Page 5. Corrosion and Archaeology
Corrosion
is one of the greatest enemies of archaeological finds and findings. All kinds
of materials are its victims: metals, glass, ceramics, mortars, stuccos, paper,
leather, textiles, pigments, dyes etc.
The kinds
of reactions causing corrosion are as numerous as the kinds of materials.
Water,
liquid or vaporous, is the mother of the overwhelming majority of corrosion
processes, supported by oxygen, carbon dioxide and other atmosperiles.
Corrosion by radiation (electromagnetic or particles) can work without water,
but humidity intensifies the attack. Carbonizing is the process of dehydration
(withdrawal of water) of cellulose.
Two special
cases of corrosion will be treated here, the electrochemical corrosion of
metals and the chemical corrosion of glass.
 The Electrochemical Corrosion of Metals
The Electrochemical Corrosion of Metals
The
electrochemical corrosion of metals is due to the formation of local galvanic
cells. The resulting electric current leads to the dissolution of the anode and
the deposition of ions on the cathode. Such galvanic cells can be formed by one
piece of metal which has two zones of different metal concentrations,
surrounded by an electrolyte, an aqueous solution. This sounds to be strange,
but the concentration of metal atoms in a piece of metal can be changed locally
by extreme pressure, as e. g. caused by hammering. Since hammering is a
frequent way of forming metal objects, this kind of corrosion happens often to
metallic archaeological finds, not only of iron, but also of bronze and other
metals. This effect makes metal plates look irregularly penetrated and if it
were eaten away.
P. C. Bol mentions briefly this phenomenon in his book
Antike Bronzetechnik – Kunst und Handwerk antiker Erzbildner, Verlag Beck
Muenchen (1985) pp. 18; 82.
|
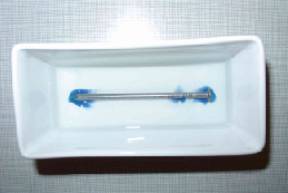
The photo
shows a beaten bronze ornamental mounting of a celtic wooden box. The typical
corrosion character can be seen. (with
kind permission of Historisches Museum Basel, CH) |
|
An instructive
experiment is outlined here which can illustrate this effect.
An iron
nail is produced by cutting off a piece of wire and forming the head and the
point by compressing and sharpening its ends, respectively, at room
temperature. Due to the extreme mechanical stress in cold condition the crystal
lattice in these zones is condensed, the concentration of iron atoms locally
increased. This leads – together with a certain roughening of the surface - to
a higher tendency to dissolve here than in zones which have not been deformed.
When immersed in an electrolyte, head and point of the nail are the anodes, the
shank is the cathode of the galvanic concentration cell formed.
The
dissolved iron, present as iron ions, can be detected by a suitable reagent. In
this case potassium hexacyanoferrate(II) has been chosen, which reacts with
iron ions to a blue compound, called Berlin Blue.
|
|
Corrosion
of an iron nail at zones compressed during production Length of
nail 80 mm. (Foto:
Author) |
The picture
given here shows a nail embedded in a gelatine gel containing a low
concentration of the reagent. You see the blue spots around head and point, indicating
that the nail starts here to dissolve. The third blue zone developed at a
little damage of the shank which caused a compression and/or damage of the
crystal lattice also here.
This
picture illustrates that compressed zones, e. g. caused by beating or
hammering, corrode easier than not compressed zones. Generally, beaten objects
corrode easier than cast objects. But be carefull: The type of alloy, the wall
thickness and the roughness of the object also play a role. Their influence
will add to the electrochemical effect.
 The Chemical Corrosion of Glass
The Chemical Corrosion of Glass
Glass is
not as resistant against attacks of corrosive media as one may think. Even
water leads to corrosion by dissolving the alkalimetal ions, preferred
potassium. Acid and alkaline substances intensify corrosion.
Antique or
medieval glass is not a homogenious material but consists of two or more
different phases (kinds of glass), which are more or less soluble in one
another. They exist side by side, mostly in form of bubbles of one phase in a
surrounding matrix of the other phase. These different kinds of glass have
different corrosion properties, one corrodes more easily than another one. This
leads to a characteristic corrosion phenomenon which is shown in the following
picture.
|
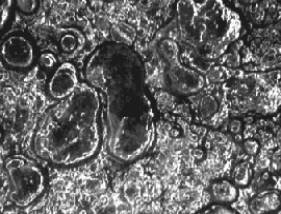
Corroded
surface of a medieval potassium glass fragment. The photo shows deepenings
formed by complete dissolution of the more soluble potassium glass phase in
the more resistant matrix. The structures are flattened by the blowing
process. (Surface microscope, magnified 15 times. The deepenings can be seen
with the naked eye). |
|
The next
photo shows the ongoing corrosion process by formation of little tiles. In a more
developed condition these tiles can be seen with the naked eye as little
silvery plates, looking like mica.
|
Corrosion
process in progress by formation of little tiles flaking off. (Scanning
electron microscope, magnified 200 times). |
|
The
relatively low corrosion resistance of potassium glass (wood-ashes glass) is
responsible for the bad condition of many medieval coloured church windows.
By the way:
also the corrosion of ceramics starts with the dissolution of potassium ions.
Literature: P. Kurzmann, Mittelalterliche Glastechnologie
(2004).